Restylane® Collection of Fillers
The Restylane® collection of fillers currently contains seven hyaluronic acid fillers, each with unique properties for tissue augmentation.
Restylane Collection of Fillers
How does Restylane Collection of Fillers work?
Our office carries the full Restylane® collection, which currently contains Restylane, Restylane Refyne, Restylane Defyne, Restylane Lyft, Restylane Kysse, Restylane Silk, Restylane Eyelight and Restylane Contour. Restylane® fillers are made of hyaluronic acid, a substance found naturally in the body, and are designed to add structure, support and symmetry to the face. Fillers have on-label approved areas, but in the hands of a skilled injector can be used elsewhere off-label. Each filler is best suited for a specific area, concern and facial anatomy, which is where experience and expertise become important.
Restylane
Restylane® was the first Hyaluronic Acid filler FDA approved in the United States. Dr. Cox was involved in many of the early clinical trials for Restylane®, beginning in the early 2000s. Originally used for line filling in the nasolabial folds and marionette lines, Restylane® was an industry disrupter, changing how many dermatologists and plastic surgeons practiced medicine. Today, Dr. Cox uses Restylane® in many areas of the face, most popularly under the eyes.
Restylane Lyft
Formerly known as Perlane, Restylane® Lyft is comprised of a larger HA gel particle than Restylane®. Restylane® Lyft is approved for the improvement of deep facial folds, midfacial rejuvenation, and the hands.
Restylane Silk
Another industry disrupter, Restylane® Silk was the first FDA-approved hyaluronic acid filler for use specifically in the lips. Restylane® Silk is a nice, smooth filler that gives subtle volume and hydration.
Restylane Kysse
It’s no surprise that Restylane® Kysse is formulated for the lips. Restylane® Kysse uses XpresHAn Technology™ and is designed to provide volume and hydration while still being natural and flexible.
Restylane Contour
The newest filler in the Restylane® collection, Contour was designed for use in the midface to restore the support lost in the natural aging process. Restylane® Contour also uses XpresHAn Technology™, and does a nice job of adding definition to the midface while also looking natural in expression.
Restylane Refyne
Restylane® Refyne is designed with XpresHAn Technology™ to soften laugh lines, although it can be used off-label in other areas. Refyne is a soft, midweight filler making it ideal for filling shallow fine lines and wrinkles.
Restylane Defyne
Restylane® Defyne is also designed with XpresHAn Technology™ to soften lines. Defyne is a “stiffer” filler than Refyne, making it ideal for areas where more support is needed. Dr. Cox will often use Defyne in the midface, chin, and jawline. It’s important to keep in mind patient anatomy is key to product selection, and not all treatment plans are the same.
Restylane Eyelight
Restylane Eyelight is Restyalne-L packaged in a dosage ideal for the undereye area. Restylane earned approval for the undereye area in May 2023. Dr. Cox has used this product in the undereye region off-label for many years.
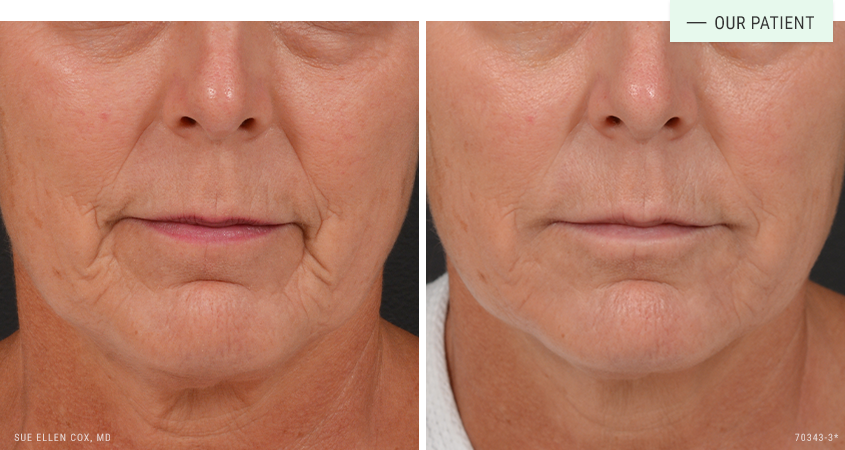
Before | After: Perioral restoration with dermal fillers. Dr. Cox used a total of 4mL spaced across multiple sessions.
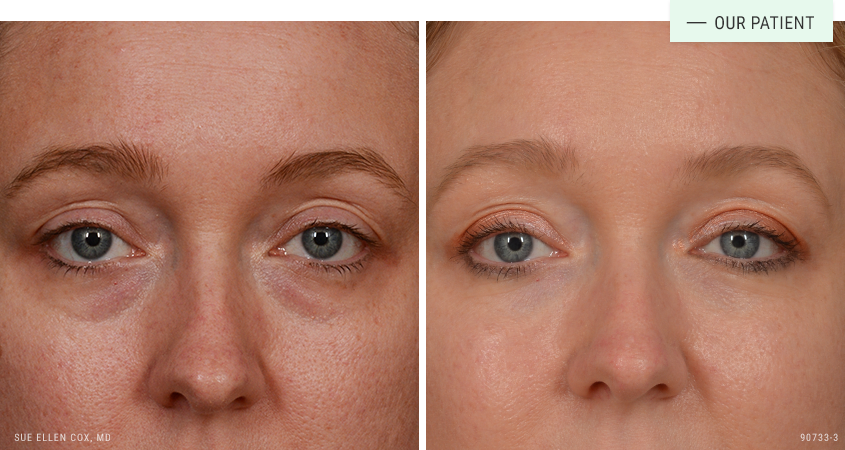
Before | After: Restylane for the undereyes. The undereyes are a high-risk injection area. Dr. Cox stresses the importance of seeing a skilled injector for this treatment area.
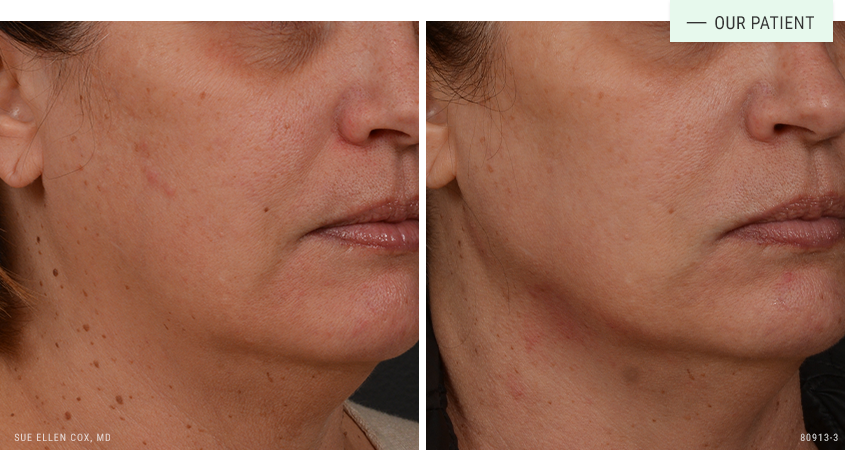
Before | After: Dermal fillers to define the jawline. This shows results after one session with 1mL. A series of sessions produce the best results.
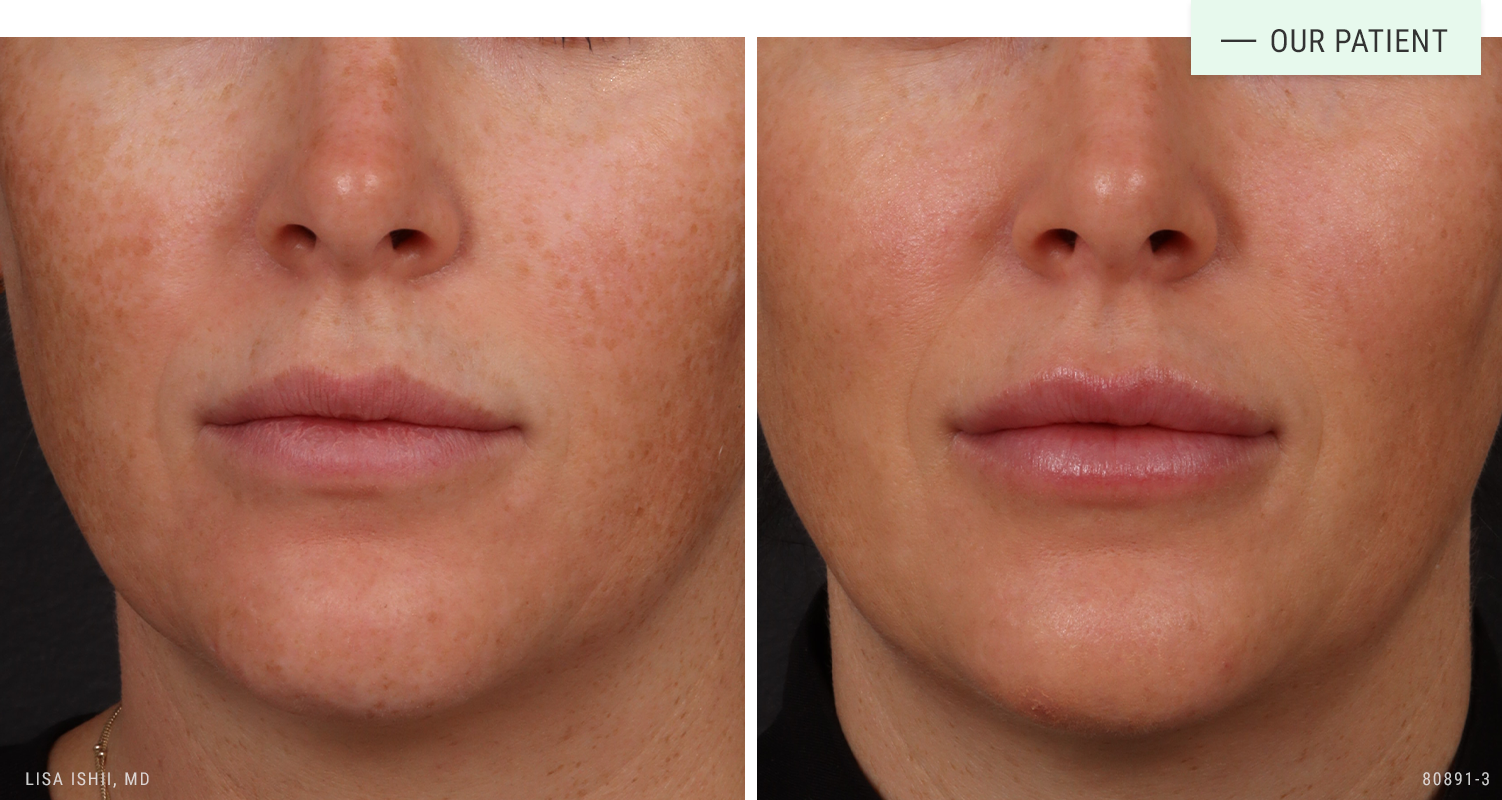
Before | After: One syringe of filler for a natural, hydrated appearance to the lips.
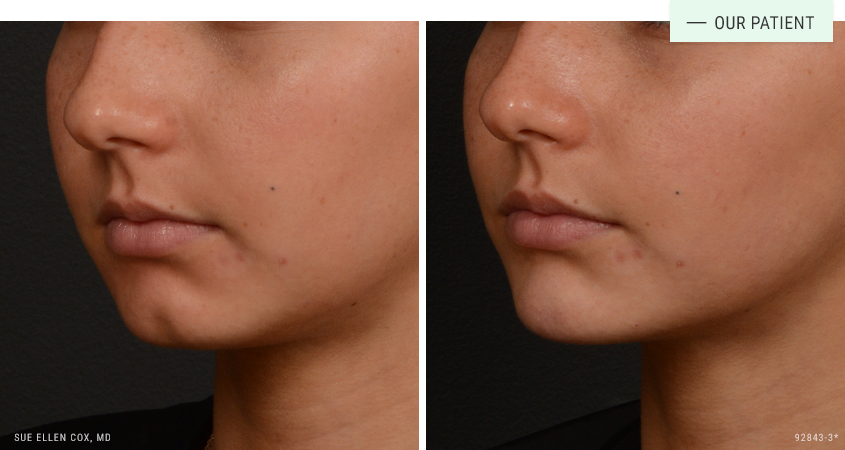
Before | After: Dermal fillers for chin augmentation and facial balancing. This shows results immediately after one session with 1mL. A series of sessions produce the best results.
Is Restylane Collection of Fillers safe?
Each Restylane® filler has an excellent safety profile and is FDA approved. Like any surgical or non-surgical procedure, there are risks associated with treatment. As an international teacher and trainer of other physicians in facial anatomy and injection techniques, Dr. Cox stresses the importance of finding an experienced physician who understands the finer points of injectable treatment.
Restylane Collection of Fillers downtime
Downtime from Restylane® fillers is minimal. The most common side effects of dermal fillers are bruising and swelling at the injection site, which typically resolves quickly. Dr. Cox will discuss in your consultation ways to minimize bruising and swelling.
Restylane Collection of Fillers results
Results and duration from Restylane® fillers vary based on the quantity, placement, and treatment area. Dr. Cox often recommends patients receive filler treatments twice yearly to maintain optimal results.
Related Clinical Trials
Lip Filler Study 1711-60
Status: Completed
Dr. Cox has completed work as principal investigator for this study “A Randomized, Controlled, Evaluator-Blinded, Multi-Center Study to Evaluate the Effectiveness and Safety of Restylane Kysse versus a Control in the Augmentation of Soft Tissue Fullness of the Lip.” Dr. Cox found this new product to be unique and effective, and we’re excited to now have Restylane Kysse as a treatment option for patients. This study began in November 2017 and ended in June of 2019.
Restylane Lip Filler 0907-14
Status: Completed
Dr. Cox was principal investigator in the study “A Randomized, Evaluator-Blinded No-Treatment-Controlled Study of the Effectiveness and Safety of Restylane® in the Augmentation of Soft Tissue Fullness of the Lips.” The study was performed July 2009 through December 2011.
Restylane Lip Filler 1205-24
Status: Completed
Dr. Cox was principal investigator in the study “A Randomized, Evaluator-Blinded, No-Treatment-Controlled Study of the Effectiveness and Safety of Small Particle Hyaluronic Acid plus Lidocaine (SPHAL) in the Augmentation of Soft Tissue Fullness of the Lips.” The study was performed May 2012 to July 2013.
Restylane & Perlane 0506-03
Status: Completed
Dr. Cox was principal investigator for the study “A Prospective, Randomized, Comparative, Multi-Center Study of Sensitization to Restylane® and Perlane® and Including an Acute Safety Profile Assessment.” The study was performed July to December 2005.
Nasolabial Filler 0811-11
Status: Completed
Dr. Cox was principal Investigator for the study “A Randomized, Double-Blind, Multi-Center Comparison of the Safety and Efficacy of Coapt Gel and Coapt Gel Plus versus Restylane for the Correction of Nasolabial Folds.” The study was performed November 2008 through November 2010.
Temple Filler 2303-97
Status: Completed
Dr. Cox completed work as Principal Investigator for the study A Randomized, no-treatment-controlled, evaluator-blinded, multi-center study to evaluate the effectiveness and safety of Restylane Contour in the treatment of temple hollowing.
Dr. Cox has long been using HA filler in the temple region to correct hollowing, improve overall facial contour and lift the brow. We hope to see this indication approved and more widely used in practice! This study began in March 2023 and ended in February 2025.
