JUVÉDERM® Collection of Fillers
The JUVÉDERM® collection of fillers contains a number of hyaluronic acid fillers designed to restore volume and support.
JUVÉDERM Collection of Fillers
How does JUVÉDERM Collection of Fillers work?
Our office carries four fillers from the JUVÉDERM® collection – JUVÉDERM Ultra, JUVÉDERM Ultra Plus, JUVÉDERM Voluma and JUVÉDERM Volbella. These are all hyaluronic acid fillers designed to restore volume, support, and symmetry. Fillers have on-label approved areas, but in the hands of a skilled injector can be used elsewhere off-label. Each filler is best suited for a specific area, concern, and facial anatomy, which is where experience and expertise become important.
JUVÉDERM® Ultra XC
JUVÉDERM® Ultra is made of a 24mg/ml cross-linked hyaluronic acid. This filler is typically used for the correction of moderate creases and folds and in the perioral area.
JUVÉDERM® Ultra Plus XC
JUVÉDERM® Ultra Plus has a thicker texture and greater viscosity to JUVÉDERM® Ultra. This makes it ideal for use in older patients with more severe folds, or where more structural support is needed.
JUVÉDERM® VOLUMA® XC
JUVÉDERM® VOLUMA® was the first FDA-approved filler made specifically for the midface. Dr. Cox performed the largest single-site clinical trials for the registration of Voluma. In addition to the midface, Dr. Cox uses VOLUMA® off-label in the chin, temples, and other areas of the face.
JUVÉDERM® VOLBELLA® XC
JUVÉDERM® VOLBELLA® is most used in and around the lips, as well as under the eyes. VOLBELLA® has a thin, soft consistency making it ideal for subtle, delicate augmentation.
JUVÉDERM® VOLUX™ XC
JUVÉDERM® VOLUX® is FDA approved for the improvement of jawline definition. Dr. Cox conducted the clinical trials for VOLUX®.
Skinvive
SKINVIVE™ by JUVÉDERM® is the first and only FDA-approved hyaluronic acid (HA) microdroplet injectable in the US to improve skin smoothness of the cheeks. Our office uses Skinvive off label for a variety of concerns, including horizontal lines on the neck, radial cheek lines and scars.
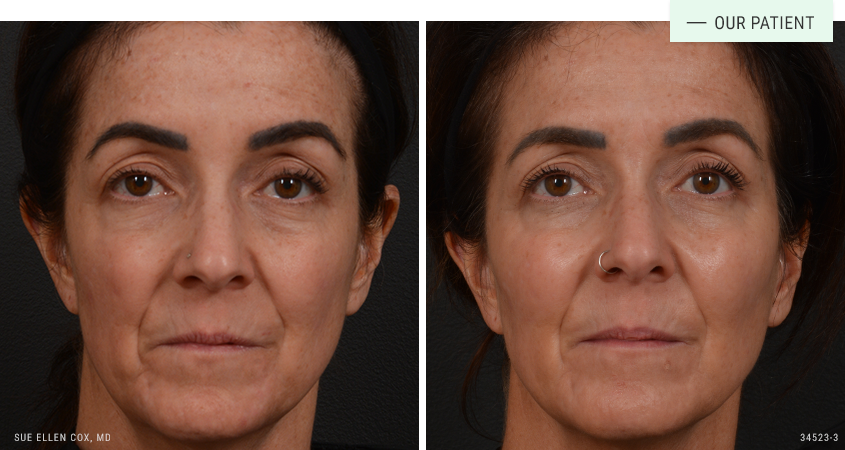
Before | After: By addressing the loss of bony support in the midface with dermal fillers, Dr. Cox is able to achieve lift. Treatment in the midface also minimized the appearance of nasolabial folds and laxity around the mouth and along the jawline.
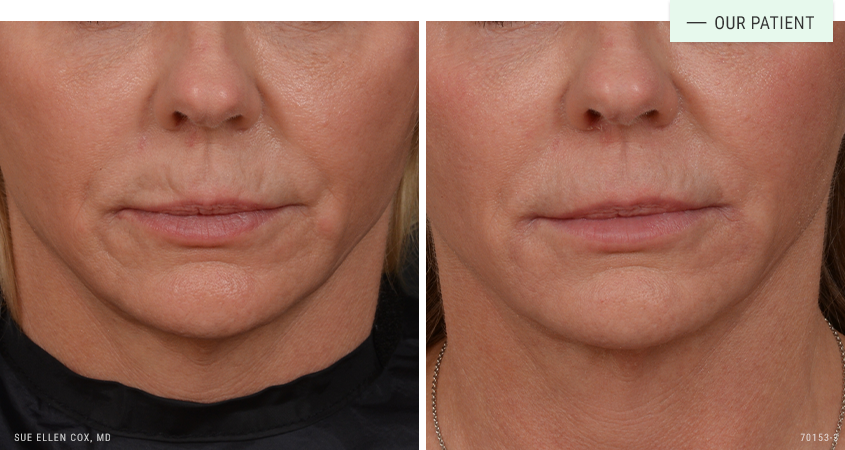
Before | After: Before and after perioral restoration with dermal fillers. A series of sessions produces the best results.
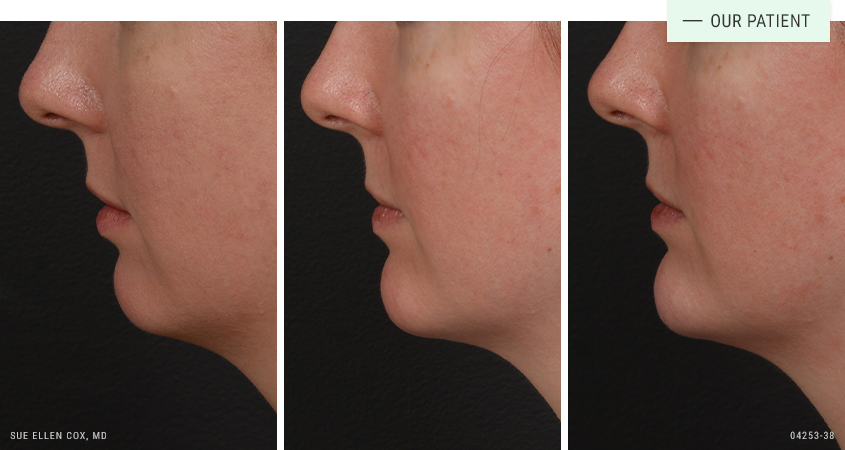
Before | After: Before and after filler to the chin for facial symmetry. A series of sessions produces the best results.

Before | After: As we age, temple hollowing becomes more prominent. Dermal fillers are able to restore volume to the temples maintaining a youthful facial shape.
Is JUVÉDERM Collection of Fillers safe?
Each JUVÉDERM® filler has an excellent safety profile and is FDA approved. Like any surgical or non-surgical procedure, there are risks associated with treatment. As an international teacher and trainer of other physicians in facial anatomy and injection techniques, Dr. Cox stresses the importance of finding an experienced physician who understands the finer points of injectable treatment.
JUVÉDERM Collection of Fillers downtime
Downtime from JUVÉDERM® fillers is minimal. The most common side effects of dermal fillers are bruising and swelling at the injection site, which typically resolves quickly. Dr. Cox will discuss in your consultation ways to minimize bruising and swelling.
JUVÉDERM Collection of Fillers results
Results and duration from JUVÉDERM® fillers vary based on the quantity, placement, and treatment area. Clinical results have demonstrated duration of four months to over two years, depending on the product.
Related Clinical Trials
Juvederm Voluma 0910-16
Status: Completed
Dr. Cox was principal investigator for the study “A Multicenter, Single-Blind, Randomized, “No-Treatment” Control, Study of the Safety and Effectiveness of JUVEDERM® VOLUMA XC Injectable Gel for Cheek Augmentation to Correct Age-Related Volume Deficit in the Mid-Face.” The study was performed October 2009 through November 2013. Over 40 Triangle residents were introduced to JUVEDERM® VOLUMA XC years before it ever came to market!
Juvederm vs Belotero 1310-31
Status: Completed
Dr. Cox was principal investigator in the study “A Prospective, Randomized, Controlled, Multi-Center, Study of the Safety and Effectiveness of JUVÉDERM® Ultra XC Injectable Gel versus Belotero Balance® for Perioral Lines.” The study compared two fillers used to treat lines around the mouth. The study was active from October 2013 through August 2015.
Cheek Filler 1802-62
Status: Completed
Dr. Cox completed work as the principal investigator on this multicenter, single-blind, randomized, controlled study of the safety and effectiveness of JUVÉDERM VOLUMA® XC injectable gel for cheek augmentation using a cannula. Dr. Cox has long been an advocate for the safety and effectiveness of injecting with the use of a cannula rather than just a needle in the mid-face. This study began in February of 2018 and finished in October of 2018.
Jawline Filler 1811-63
Status: Completed
Dr. Cox has completed work as the principal investigator for this multicenter, evaluator-blinded, randomized, parallel-group, controlled study of the safety and effectiveness of JUVÉDERM VOLUX™ XC injectable gel for restoring jawline definition. This study began in November 2018 and ended in April 2021. VOLUX earned FDA approval in August 2022.
Under Eye Filler 1802-61
Status: Completed
Dr. Cox has completed work as principal investigator for this study “A Multicenter, Single-Blind, Randomized, Controlled Study of the Safety and Effectiveness of JUVÉDERM VOLBELLA for Correction of Infraorbital Hollowing.” Dr. Cox was pleased with the beautiful, natural results achieved. This study began in February 2018 and ended in October 2019.
Temple Filler 2005-68
Status: Completed
We have completed this study evaluating JUVÉDERM VOLUMA® XC for the correction of temple hollowing. Dr. Cox served as the principal investigator, and was very pleased with results from this trial. We hope to see the temples as an on-label indication in the coming years. This study began in May 2020 and ended in November 2022.
Juvéderm Volite (Skinvive) for Transverse Neck Lines 2204-80
Status: Completed
Dr. Cox served as Principal Investigator in this Multicenter, Evaluator-blinded, Randomized, Controlled Study of the Safety and Effectiveness of JUVÉDERM® VOLITE™ XC Injectable Gel for Improvement in Neck Appearance. This study began April 2023 and ended January 2024. Juvéderm Volite (known as Juvéderm Skinvive in the United States) brought nice results for some patients when a series of sessions was conducted. A proper patient evaluation of anatomy and skin quality will be key for determining candidacy.
